Having spent yesterday at work I thought I'd spend this afternoon relaxing, not only have I rediscovered a childhood favourite - the N64 (I'm currently working my way through Zelda -Ocarina of Time), but I've also found some time to sit and have a quick look through this months Chemistry World. They've been stacking up to be honest, they always seem to be delivered when we're in the midst of a coursework crisis and extra chemistry is the last thing I want to do, but now I've had a few weeks off uni I thought I'd have a read through.
Having been a member of chemnet during sixth form, and now a student member of the RSC I've been receiving chemistry world for over 3 years and its amazing how much more I understand now. The issues I read during my a-levels made little sense, with just the odd article being about something I knew about. Although I'd still read it, as it was always quite interesting anyway! Now there is so much more that I know, I tend to just flick through and read a couple of articles rather than going through the whole thing cover to cover but there are aways some interesting stories.
Something that caught my eye today is perhaps not the most ground breaking or exciting article in the magazine but definitely worth a read for the budding organic chemist. It is about the synthesis of a molecule called "conophylline" which has potential as a treatment for tyre 1 diabetes as well as playing a possible therapeutic role in pancreatic cancer.
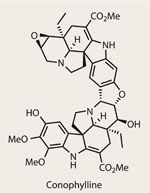
I'm not going to lie, although I could maybe come up with some sort of retrosynthesis and possibly a forward synthesis it would most likely be incomplete and definitely wouldn't be the most efficient way to do it, but as a second year (very nearly third year) I accept that, because I just haven't practised enough yet. But this article also provides the steps of the reaction, many of which are the sort of reactions that I have carried out myself (just on less complicated molecules). Although I can't quite follow everything that's going on there are things like a Diels-Alder, a Mannich and Michael reaction, as well as the use of mCPBA
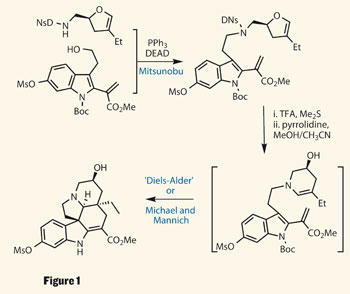
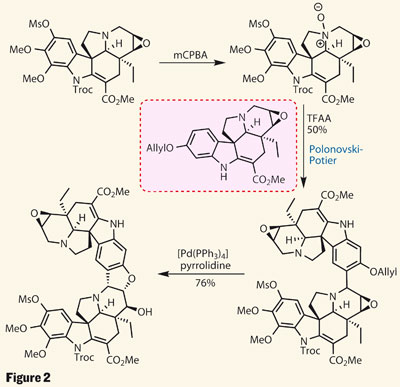
Seeing something like this really shows that the hours we have spent during curly arrows and learning what seems like an endless amount of reaction mechanisms is actually applicable to real world scenarios. So I may have slightly missed the point of the article, which was to do with the use of the Polonovski-Potier reaction but it has got me thinking about organic chemistry in my summer holiday, so thats something right?
The article can be seen here http://www.rsc.org/chemistryworld/Issues/2011/June/ColumnTotallySynthetic.asp , its also where I have taken the diagrams from as I didn't fancy redrawing them!
No comments:
Post a Comment